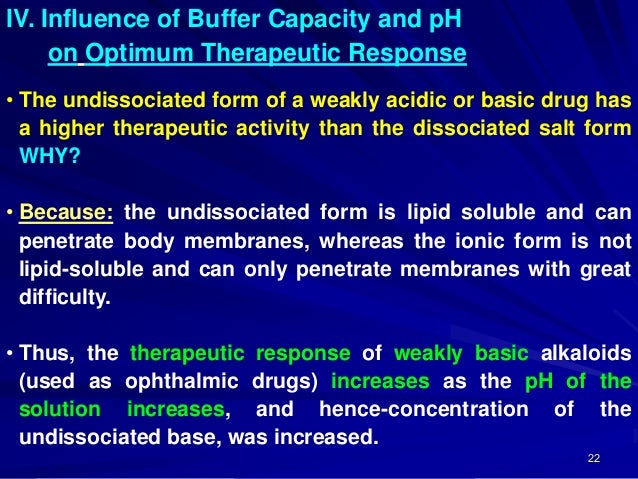
Simple Definition Of Buffer Capacity
A titration curve visually demonstrates buffer capacity where the middle part of the curve is flat because the addition of base or acid does not affect the pH of the solution drastically. Buffer capacity is a quantitative measure of the resistance to change of pH of a solution by camerino italy containing a buffering agent with respect to a change of acid or alkali concentration.
 Key Actions To Improve Supply Chain Performance Insights From Hp In Mit Sloan Management Review Change Management Supply Chain Data Analysis
Key Actions To Improve Supply Chain Performance Insights From Hp In Mit Sloan Management Review Change Management Supply Chain Data Analysis
I will define significant change as 1 pH unit.
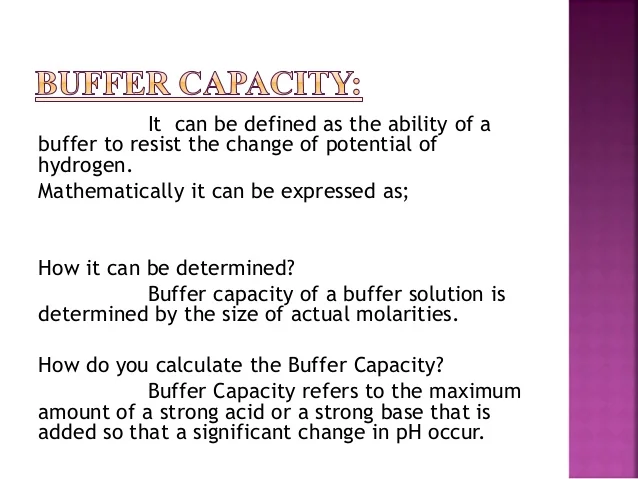
Simple definition of buffer capacity. Buffer Capacity Buffers are characterized by the pH range over which they can maintain a more or less constant pH and by their buffer capacity the amount of strong acid or base that can be absorbed before the pH changes significantly. Conventionally the buffer capacity is expressed as the amount of strong acid or base in gram-equivalents that must be added to 1 liter of the solution to change its pH by one unit. Buffer capacity is the measure of the efficiency of the buffers.
The buffer index which is the same for addition of ei-ther a strong base or a strong acid depends on r and C. A buffer resists changes in pH due to the addition of an acid or base though consumption of the buffer. The buffer capacity can also be defined as the amount of mole of strong base needed to change the pH of 1 L of solution by 1 pH of unit.
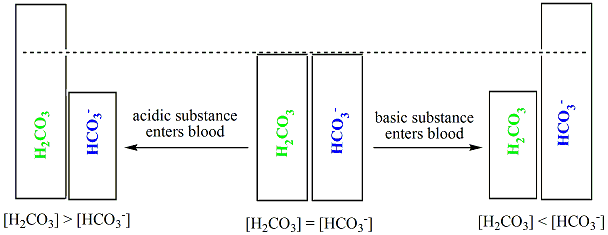
HCO₃ H₂O H₃O CO₃². Buffer capacity modeling Buffers are analogous to a water reservoir to protect against floods or drought Weak acids and bases are naturally present in acidacidified foods Protect against pH changes at pH values near pK value Many buffers in foods are not clearly identified ID buffers in low acid foods from BC curves. That is when pH pKa.
Buffer definition is - fellow man. β d C b d p H displaystyle beta frac dC_ b d mathrm pH where. The buffer capacity is defined as the amount of acid or base you can add without changing the pH by more than 1 pH unit.
A buffer is strong when both components are in equal concentrations. Definition 1 The buffer capacity of a solution is the amount in mol of strong acid or strong base that must be added to one liter of solution to change either the pH or pOH by one unit. Buffer capacity is a measure of the efficiency of a buffer in resisting changes in pH.
Let us now determine the expressions for the buffer ca-pacity of a simple buffer according to IUPAC definition when either a strong acid or a strong base is added. It reaches its maximum value β 057C when r 1 Van Slyke 1. The buffer capacity is used in the quantitative chemical analysis and the buffer index in studying biological systems.
Buffer capacity tells us how strong the buffer is in terms of withstanding any addition of base or acid. It is optimal when the ratio is 11. Where n is some equivalents of added strong base per 1 L of the solution.
Note that the addition of n moles of acid will change the pH by the same value but in the opposite direction. To begin the derivation consider the Henderson-Hasselbalch representation for eqs 1 and 2. So to give a more clear definition buffer capacity may be defined as the quantity of a strong acid or strong base that must be added to one liter of a solution to change it by one pH unit.
To elaborate if we take acid or base and add it to a buffer system there will be a change in the pH. The buffer capacity equation is as follows. A buffer is an aqueous solution used to keep the pH of a solution nearly constant.
Buffer capacity β is defined as the moles of an acid or base necessary to change the pH of a solution by 1 divided by the pH change and the volume of buffer in liters. This depends on two things the buffer ratio and the actual concentrations of the two components. The higher the acid concentration of the buffer then the buffer capacity will be higher as well.
2012 Farlex Inc. How to use buffer in a sentence. 4 where represents the acidic strength of HA.
The change can be either large or small. The buffering region is about 1 pH unit on either side of the pK a of the conjugate acid. 1 pH pKₐ log CO₃² HCO₃ pKₐ log 050035 pKₐ 0155.
It is a unitless number. It is expressed as the amount of strong acid or base that must be added to 1 liter of the solution to change its pH by one unit. The buffer capacity is numerically expressed to be equal with the minimum concentration of strong acid or strong base which causes the variation of buffers pH with one unit.
The field of application of both notions is different. Buffer capacity A measure of the resistance of a buffer solution to pH change upon addition or removal of hydroxide ions. Buffer capacity can be defined as the ability of a solution to resist rapid changes in pH.
A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. The solution either absorbs or removes H and OH- ions. The buffer capacity is a quantity in resisting the pH change at the time of addition of an acid or base.
Use this simple calculator for buffer capacity calculation of buffers. This happens only if pH pKa or 14-PKb. Calculate the buffer capacity as.
It can be defined as follows. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes.
 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
 Buffer Capacity Definition Examples Diagrams
Buffer Capacity Definition Examples Diagrams
 What Is Buffering A Simple Buffering Definition Bandwidth Place
What Is Buffering A Simple Buffering Definition Bandwidth Place
 How To Build Resilience Experiments In Wellness Emotional Resilience How To Build Resilience Coping Skills
How To Build Resilience Experiments In Wellness Emotional Resilience How To Build Resilience Coping Skills
 Pre And Post Pi Planning Scaled Agile Framework Agile Software Development Agile Project Management Lean Enterprise
Pre And Post Pi Planning Scaled Agile Framework Agile Software Development Agile Project Management Lean Enterprise
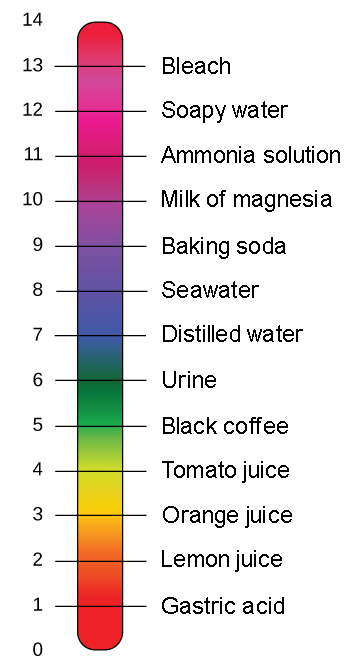 Buffers Ph Acids And Bases Biology For Non Majors I
Buffers Ph Acids And Bases Biology For Non Majors I
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
 Pin On Chemistry Acids And Bases
Pin On Chemistry Acids And Bases
 What Is A 5 Whys Buffer Stories Business Process Management Problem Solving Activities Problem Solving
What Is A 5 Whys Buffer Stories Business Process Management Problem Solving Activities Problem Solving
 Social Media Marketing Can Be Daunting Instead Of Shying Away From It In 2020 Marketing Strategy Social Media Social Media Marketing Social Media Marketing Services
Social Media Marketing Can Be Daunting Instead Of Shying Away From It In 2020 Marketing Strategy Social Media Social Media Marketing Social Media Marketing Services
 Buffer Capacity Video Buffer Solutions Khan Academy
Buffer Capacity Video Buffer Solutions Khan Academy
 Henderson Hasselbalch Equation Microbe Notes
Henderson Hasselbalch Equation Microbe Notes
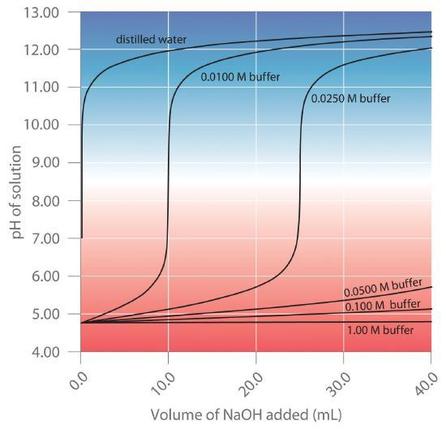 8 9 Buffer Capacity And Buffer Range Chemistry Libretexts
8 9 Buffer Capacity And Buffer Range Chemistry Libretexts